Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP (Cat. No. 420502)
For Research or Further Cell Manufacturing Use Only
Introducing Cell-Vive T-NK Xeno-Free Serum Substitute for the culture and expansion of T and NK cells. This GMP manufactured serum substitute is optimally formulated and intended to replace human AB serum, providing you more control over culture conditions with exceptional cell expansion performance. Each lot undergoes extensive QC testing to ensure consistent product activity and safety.
At BioLegend, we understand the critical steps involved in advancing your research from the lab to therapeutics. Our GMP Cell Culture Reagents drive cell expansion and enrichment to meet your cell processing needs. Our expanding line of GMP tools are manufactured under strict regulatory guidelines and are available at affordable prices. BioLegend is ISO 13485:2016 certified, and our GMP facility is ISO 13485:2016 and MDSAP certified.
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP (Cat. No. 420502)
For Research or Further Cell Manufacturing Use Only
Features:
Each lot of Cell-Vive™ T-NK Xeno-Free Serum Substitute undergoes extensive QC testing, including:
Check out Dr. Vanda Lopes’ (BioLegend) presentation “A Novel Serum Substitute for Optimal Culture of T & NK Cells” from the ISCT 2021 Virtual Annual Meeting.
Browse through our data below for the Cell-Vive™ T-NK Xeno-Free Serum Substitute featured in Dr. Lopes’ talk.
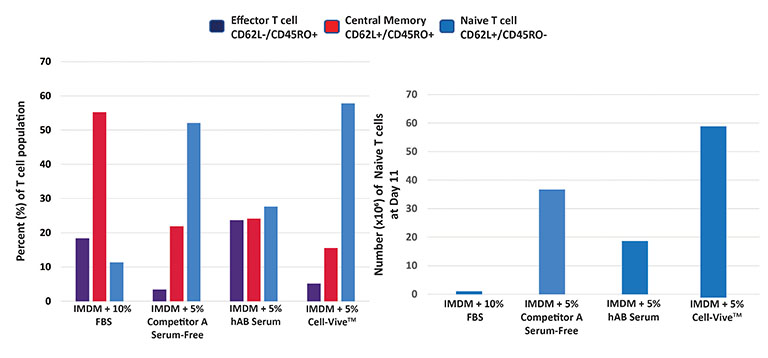
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP, supports greater cell expansion of naïve T cells, generating a higher number per million of PBMCs plated by day 11.
Procedure: PBMC-derived T cell culture was activated with anti-human CD3, anti-human CD28, and 200 IU/mL of recombinant human IL-2 using IMDM as basal media.
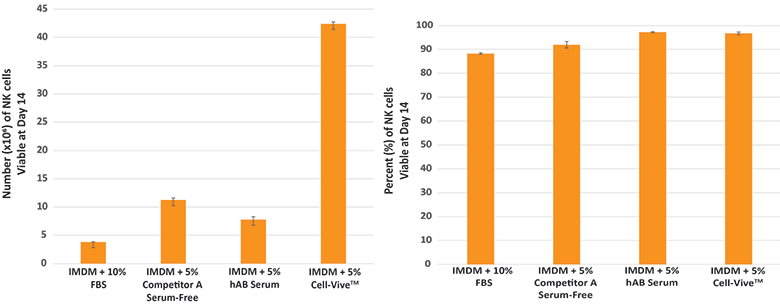
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP, supports NK cell expansion with high cell viability by day 14.
Procedure: PBMC-derived NK cells were cultured using K-562 feeder cells (2:1) and 1000 IU/mL of recombinant human IL-2 using IMDM as basal media.
 |
|---|