Enzyme Immunoassay for the Quantitative Determination of AAV Serotypes 1, 2, 3, 5, 6, 8, 9, and rh10 Particles in Cell Culture Supernatants and Purified Virus Preparations. Immunotitration by PROGEN’s AAV Titration ELISA offers a fast, sensitive and reproducible method for titration of intact AAV wild type virions, AAV recombinant virions or assembled and intact empty AAV capsids.
Principle of the AAV Titration ELISA
The principle of the assay is based on the sandwich ELISA technique. A monoclonal antibody specific for a conformational epitope on assembled AAV capsids is coated onto microtiter strips and is used to capture AAV particles from the specimen. Captured AAV particles are detected in two steps. First a biotin-conjugated monoclonal antibody to AAV is bound to the immune complex. In the second step, streptavidin peroxidase conjugate reacts with the biotin molecules. Addition of substrate solution results in a color reaction which is proportional to the amount of specifically bound viral particles. The absorbance is measured photometrically at 450 nm.
The ELISA capture antibodies
The capture antibodies used for PROGEN’s AAV ELISAs bind specific and defined conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes. Changes in the protein sequences of the capsid proteins e.g. in shuffeled vectors might influence the conformation of the proteins, hence the conformation of the antibody binding epitopes presented on the AAV capsid. Thus, influence the binding affinity of the antibody and affect determination of the titer based on the (non-shuffled) Kit Control provided with the AAV ELISA kit. A first indication that the ELISA might recognize your shuffled AAV vector is the presence of the antibody-binding epitope.
Conclusion
PROGEN’s AAV ELISAs are reliable quantification tools for total AAV capsid determination for gene therapy research and development. They are robust and accurate tools, which have been demonstrated to show low inter- and intra-assay variability. For application of AAV vectors in gene therapy, the determination of total capsid titer is essential, since most of the AAV preparations contain a significant number of empty capsids. Accurate characterization and quantification of purified AAV particle preparations represent a critical step for clinical application, to minimize immunogenic responses and maximize gene transfer to the target cells. Therefore, reliable and reproducible quantification of AAV titers is essential for safe and effective AAV gene therapy.
Currently Used Methods for AAV Titer Determination
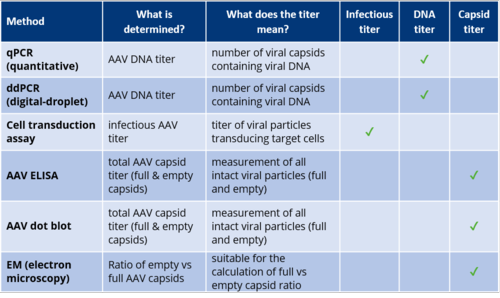
Quantification methods for rAAV vector preparations include quantitative PCR (qPCR), digital droplet PCR (ddPCR) for measuring DNA, dot blot, and enzyme-linked immunosorbent assay (ELISA) for intact viral capsids proteins. Other methods using protein chromatography, flow cytometry, or virus particle counting instruments have also been described, but are generally only applicable for highly skilled users and/or require specialised and expensive equipment. Electron microscopy can be used to determine the ratio of empty and full viral capsids, but is not useful for an absolute quantification of particles.
Comparison of AAV Quantification Methods
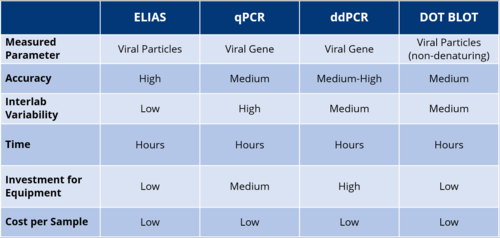
Each of the mentioned techniques has its pros and cons: qPCR is widely used, but suffers from several issues such as sample preparation, primer design, or PCR efficiency that can lead to high inter-laboratory variation of results for identical samples. Digital droplet PCR methods overcome some of the limitations of qPCR. There is no need of a dilution series of standard DNA with known concentrations to measure an unknown sample, and limited PCR-efficiency is not an issue as it is for qPCR. However, variations between labs can still occur due to different sample processing protocols. Dot blot is a relatively simple and quantitative method, but works only with reliable reference material, while suffering from the limited linearity and dynamic range of western blotting in general.
Given the practical drawbacks of the aforementioned techniques, a conventional sandwich ELISA currently appears to be the best format for the quantification of rAVV preparations.
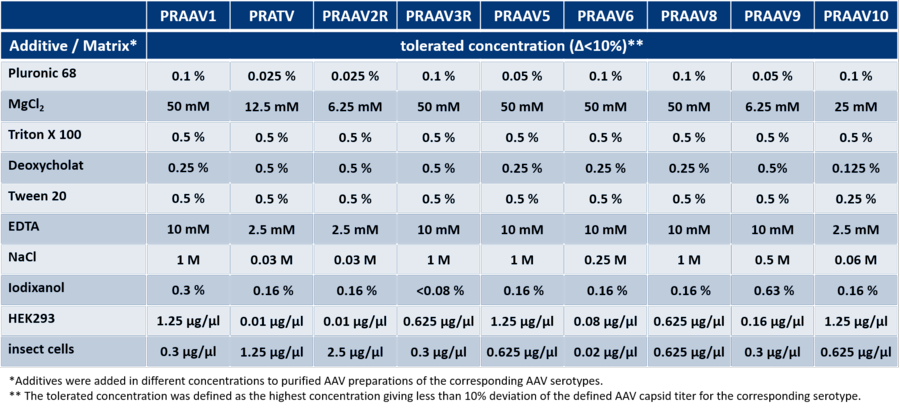
Matrix Effects of additives on the AAV Titration ELISA
The determination of AAV capsid titers from e.g. cell extract can be influenced by several conditions such as the composition of your lysis buffer. For example, high salt concentrations in your buffer might inhibit adequate capsid detection. For more information, refer to to the table below showing the analysis of matrix effects of different additives on the AAV ELISAs. Please note, that data on cumulative effects have not been collected therefore tolerated concentrations might differ from the concentrations indicated in the table.