There is an increasing interest towards immunotherapies in the field of oncology, as a result of excellent response to treatments. For this reason, pharmaceutical companies have many novel immuno-oncological drugs in their pipelines. Many of the developing drug candidates are monoclonal antibodies, that are specific for one immuno checkpoint. These novel immunotherapies are based on the specific antibody that blocks tumor antigen and it`s ligand interaction, which stimulates and enhances the patient`s immune system to recognize tumor cells.
Signaling and interactions between cancerous cells and immune system is a complex mechanism, which offers multiple possibilities to develop and optimize individual immunotherapies. The work towards individualized and better patient care has started and is showing good results.
BioSiteHisto – methods & results
We at BioSiteHisto are in the front line in developing preclinical and clinical immunohistochemical (IHC) assays for drug candidates. Our sophisticated and tailored methods offer reliable results in well-controlled conditions. Our GLP compliance test facility with fully open and flexible staining platforms offer high quality standard, especially in preclinical histopathological IHC-studies.
BioSiteHisto`s massive repertoire of optimized antibodies for human and mouse cells allow us to tailor the best possible studies in the field of immune-oncology. We have human specific as well as mouse specific T-cell antibodies, as an example for CAR-T -studies. We also have excellent markers for tumor infiltrating lymphocytes, which are crucial to detect with correct and controlled methods. Also, our markers for immune checkpoint pathways (eg. PD-L1 and PD1, TIM3 etc.) or markers for T- and B-cell activation offer great possibility to offer multiplexing IHC -assays with chromogenic, fluorogenic or combination with these detection methods.
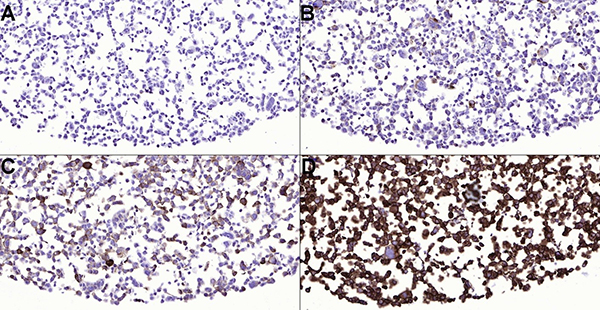

Figure 1. PD-L1 stained tonsil (A) and non-small cell lung carcinoma (NSCLC) (B-D). Tissue sections have been stained with PD-L1 (ZR3) antibody and 1:150 dilution. Tonsil is a good control for PD-L1 staining and epithelial crypt cells are strongly stained. Macrophages of germinal center must have weak to moderate membranous staining reaction in optimal staining result. Note different expression patterns of NSCLC sections (B-D). Case B is PD-L1 negative and tumor proportion score (TPS) in case C is 1-49 %. Note alveolar macrophages and weak heterogenous staining of pulmonary carcinoma. TPS is >50 % in case D and the expression of the PD-L1 is high.
Figure 2. PD-L1 stained cell pellet sections (A-D). Note different PD-L1 expression levels of the cells. Together with the tonsil, cell pellets are good control material for PD-L1 immunohistochemical assays. FFPE cell pellet sections have been stained with PD-L1 (ZR3) antibody and 1:150 dilution.